Our Plan
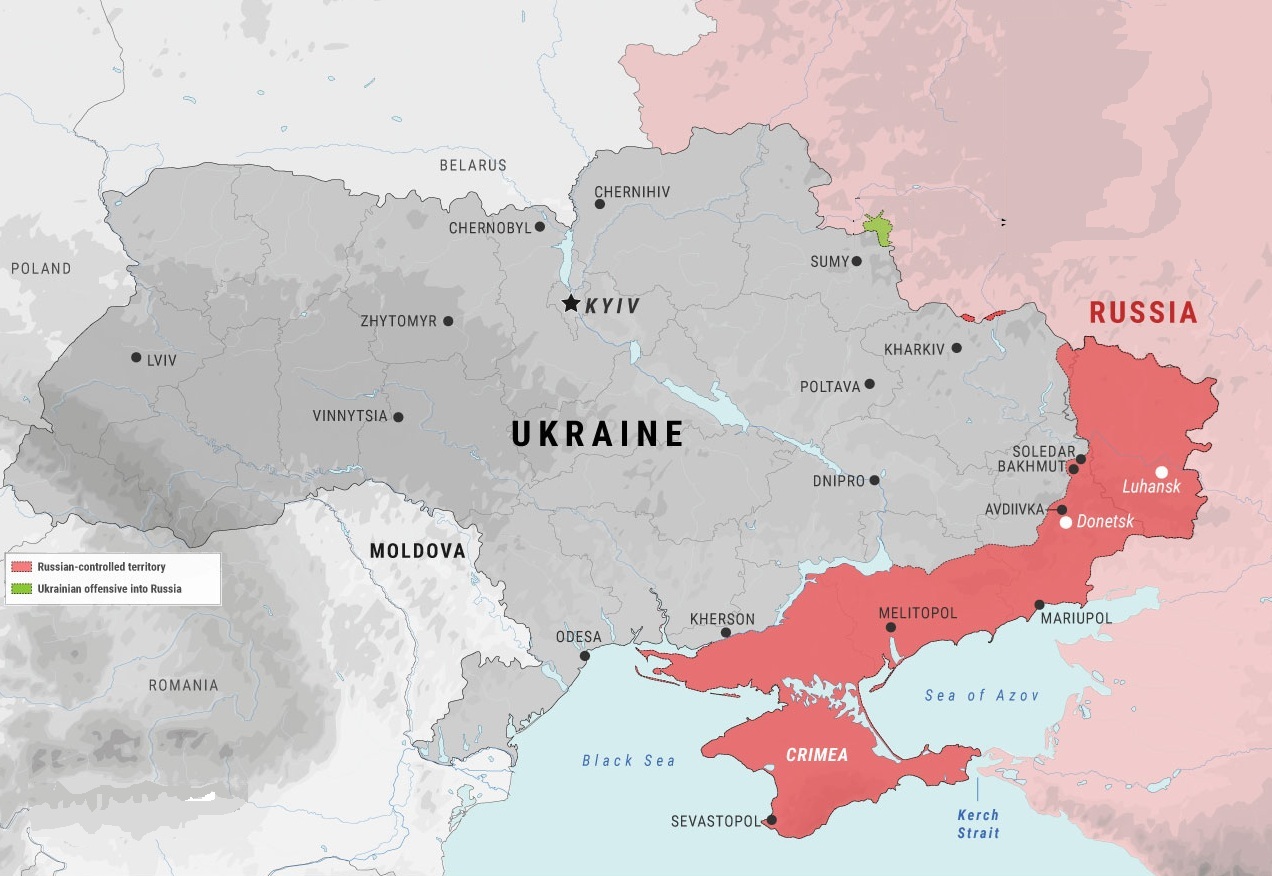
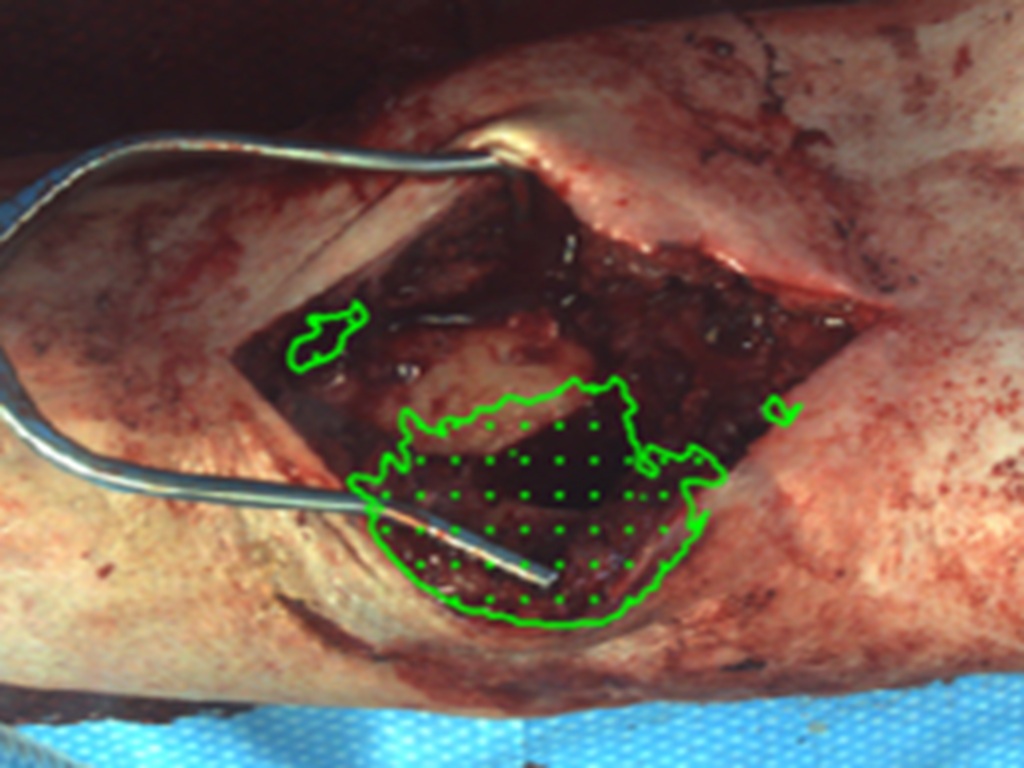
The Lumine Trauma Foundation aims to equip Ukrainian surgeons with Fluorescence Guided Debridement (FGD) technology, a portable, USB-powered device that enhances precision in debridement surgeries. These surgeries are vital for preventing infection and amputation in up to 75% of injured civilians and soldiers in Ukraine with extremity fractures. By supporting the deployment of FGD devices to Ukraine’s front lines, donors will help improve patient outcomes by enabling surgeons to more effectively identify and remove dead tissue, reducing risks of infection, non-union, and amputation. The Foundation's plan includes:
01 Deployment
Building and sending portable, rugged, battery-powered imaging systems to partner hospitals in Dnipro and Kharkiv following FDA 510(k) clearance.
02 Training
Virtual training sessions on using FGD and interacting with the user interface for surgeon-driven design changes.
03 Collaboration
Working with surgeons in Eastern Ukraine, the Ukrainian State Health Department, and local IRB.
04 Follow-Up
Patients are imaged before and after debridement, tracking outcomes like infection and amputation rates.
05 Report Findings
Publishing data in academic journals and sharing progress with contributors.
06 Feedback
Ukrainian surgeons provide feedback as a collaborative team to improve and adjust the technology.